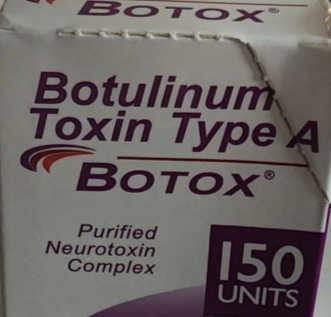
A package deal of counterfeit Botox.
At the least 19 ladies throughout 9 US states seem to have been poisoned by bogus injections of Botox, the Facilities for Illness Management and Prevention reported late Monday.
9 of the 19 circumstances—47 %—have been hospitalized and 4—21 %—have been handled with botulinum anti-toxin. The CDC’s alert and outbreak investigation follows studies in current days of botulism-like diseases linked to shady injections in Tennessee, the place officers reported 4 circumstances, and Illinois, the place there have been two. The CDC now studies that the record of affected states additionally consists of: Colorado, Florida, Kentucky, Nebraska, New Jersey, New York, and Washington.
In a separate alert Tuesday, the Meals and Drug Administration stated that “unsafe, counterfeit” variations of Botox had been present in a number of states, and the poisonous fakes have been administered by unlicensed or untrained folks and/or in non-medical or unlicensed settings, corresponding to properties or spas. The counterfeit merchandise appeared to have come from an unlicensed supply, typically elevating the dangers that they are “misbranded, adulterated, counterfeit, contaminated, improperly saved and transported, ineffective and/or unsafe,” the FDA stated.
The CDC and the FDA listed the varied signs that adopted injections of the counterfeit Botox, which embrace: blurred or double imaginative and prescient, drooping eyelids, problem swallowing, dry mouth, slurred speech, constipation, incontinence, shortness of breath or problem respiratory, weak point, and problem lifting one’s head. “These signs are much like these seen when botulinum toxin spreads to different components of the physique,” the FDA wrote. Anybody experiencing these signs after an injection ought to go to the emergency room or contact a well being care skilled.
Botox is a regulated drug containing purified, managed doses of botulinum toxin, a neurotoxin made by Clostridium micro organism that causes muscle paralysis by blocking a neurotransmitter. It is typically injected into the face to cut back the looks of wrinkles. The CDC reported that every one 19 circumstances recognized thus far are in ladies between the ages of 25 and 59. Eighteen of the 19 particularly reported getting the injections for beauty functions.
However dangerous publicity to the toxin—corresponding to from an an infection, consuming contaminated meals, or use of counterfeit Botox—could cause botulism or at the very least botulism-like diseases. In extreme circumstances, botulism can progress to descending, symmetric muscle weak point, full muscle paralysis, and might generally be deadly. The CDC reported that a few of the folks within the outbreak have been hospitalized and handled with anti-toxin out of concern that the toxin had unfold past the injection website. Nevertheless, the company famous that 5 folks have been particularly examined for botulism, and all examined damaging.
In an e mail to Ars late final week, the CDC advisable that anybody taken with a Botox injection achieve this utilizing “an FDA-approved product, administered by licensed suppliers and in licensed settings.” The company added in its alert Monday: ” If unsure, don’t get the injection.”
The FDA, in the meantime, offered detailed info on how to make sure your shot of Botox is the true factor. FDA-approved Botox is made by AbbVie, and genuine Botox merchandise are available unit doses of fifty, 100, and 200. The surface of the field ought to say “BOTOX® COSMETIC / onabotulinumtoxinA / for Injection” or “OnabotulinumtoxinA / BOTOX® / for injection,” and it ought to record the producer as both “Allergan Aesthetics / An AbbVie Firm” or “abbvie.” The energetic ingredient needs to be listed as “OnabotulinumtoxinA” on the field.
In distinction, a few of the counterfeit variations the FDA has tracked down thus far have been bought in 150-unit doses (not made by AbbVie), solely seem to have “Allergan” on the field (not the total producer title), and the energetic ingredient is displayed as “Botulinum Toxin Kind A” as an alternative of “OnabotulinumtoxinA.” The counterfeit variations even have had non-English language textual content on the skin of the field and displayed lots variety of C3709C3. Any considered one of these options is an indication that the product is counterfeit. Pictures of the counterfeit merchandise from the FDA are under.

