
The US Division of Agriculture this week posted an unpublished model of its genetic evaluation into the spillover and unfold of chicken flu into US dairy cattle, providing probably the most full look but on the knowledge state and federal investigators have amassed within the surprising and worrisome outbreak—and what it’d imply.
The preprint evaluation offers a number of important insights into the outbreak—from when it might have truly began, simply how a lot transmission we’re lacking, beautiful unknowns about the one human an infection linked to the outbreak, and the way a lot the virus continues to evolve in cows. The data is vital as flu specialists worry the outbreak is heightening the ever-present threat that this wily flu virus will evolve to unfold amongst people and spark a pandemic.
However, the knowledge hasn’t been straightforward to return by. Since March 25—when the USDA confirmed for the primary time {that a} herd of US dairy cows had contracted the extremely pathogenic avian influenza H5N1 virus—the company has garnered worldwide criticism for not sharing knowledge rapidly or utterly. On April 21, the company dumped over 200 genetic sequences into public databases amid strain from exterior specialists. Nonetheless, a lot of these sequences lack descriptive metadata, which usually accommodates primary and key bits of data, like when and the place the viral pattern was taken. Exterior specialists do not have that essential info, making impartial analyses frustratingly restricted. Thus, the brand new USDA evaluation—which presumably consists of that knowledge—gives the very best but glimpse of the whole info on the outbreak.
Undetected unfold
One of many massive takeaways is that USDA researchers suppose the spillover of chicken flu from wild birds to cattle started late final 12 months, seemingly in December. Thus, the virus seemingly circulated undetected in dairy cows for round 4 months earlier than the USDA’s March 25 affirmation of an an infection in a Texas herd.
This timeline conclusion largely aligns with what exterior specialists beforehand gleaned from the restricted publicly accessible knowledge. So, it might not shock these following the outbreak, however it’s worrisome. Months of undetected unfold elevate important considerations concerning the nation’s capacity to establish and swiftly reply to rising infectious illness outbreaks—and whether or not public well being responses have moved previous the missteps seen within the early phases of the COVID-19 pandemic.
However one other massive discovering from the preprint is what number of gaps nonetheless exist in our present understanding of the outbreak. Thus far, the USDA has recognized 36 herds in 9 states which have been contaminated with H5N1. The excellent news from the genetic evaluation is that the USDA can draw traces connecting most of them. USDA researchers reported that “direct motion of cattle based mostly upon manufacturing practices” appears to clarify how H5N1 hopped from the Texas panhandle area—the place the preliminary spillover is assumed to have occurred—to 9 different states, some as far-flung as North Carolina, Michigan, and Idaho.
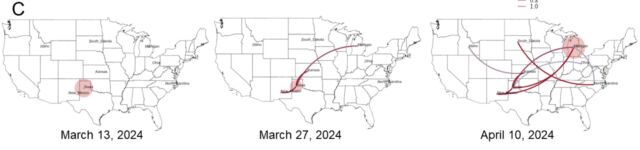
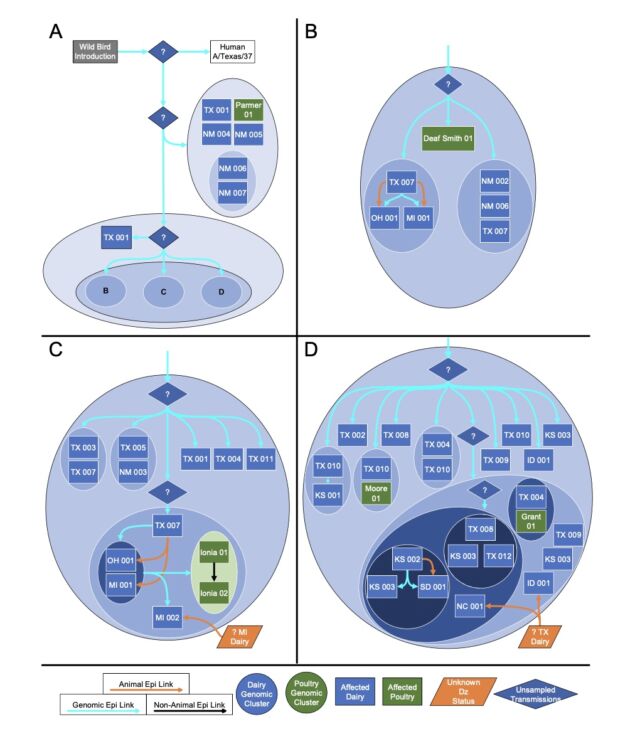
Putative transmission pathways of HPAI H5N1 clade 2.3.4.4b genotype B3.13 supported by epidemiological hyperlinks, animal actions, and genomic evaluation. [/ars_img]The dangerous information is that these traces connecting the herds aren’t stable. There are gaps during which the genetic knowledge suggests unidentified transmission occurred, perhaps in unsampled cows, perhaps in different animals completely. The genetic knowledge is obvious that after this pressure of chicken flu—H5N1 clade 2.3.4.4 genotype B3.13 —hopped into cattle, it may readily unfold to different mammals. The genetic knowledge hyperlinks viruses from cattle transferring many occasions into different animals: There have been 5 cattle-to-poultry jumps, one cattle-to-raccoon transmission, two occasions the place the virus moved from cattle to home cats, and 3 times when the virus from cattle spilled again into wild birds.
“We can not exclude the likelihood that this genotype is circulating in unsampled areas and hosts as the prevailing evaluation means that knowledge are lacking and undersurveillance could obscure transmission inferred utilizing phylogenetic strategies,” the USDA researchers wrote of their preprint.

